Introduction
Atoms are the fundamental units of matter, and elements consist of atoms.But not all atoms are the same, even within the same element. the concepts of isotopes and isobars.
Difference between isotopes and isobars [in Table]
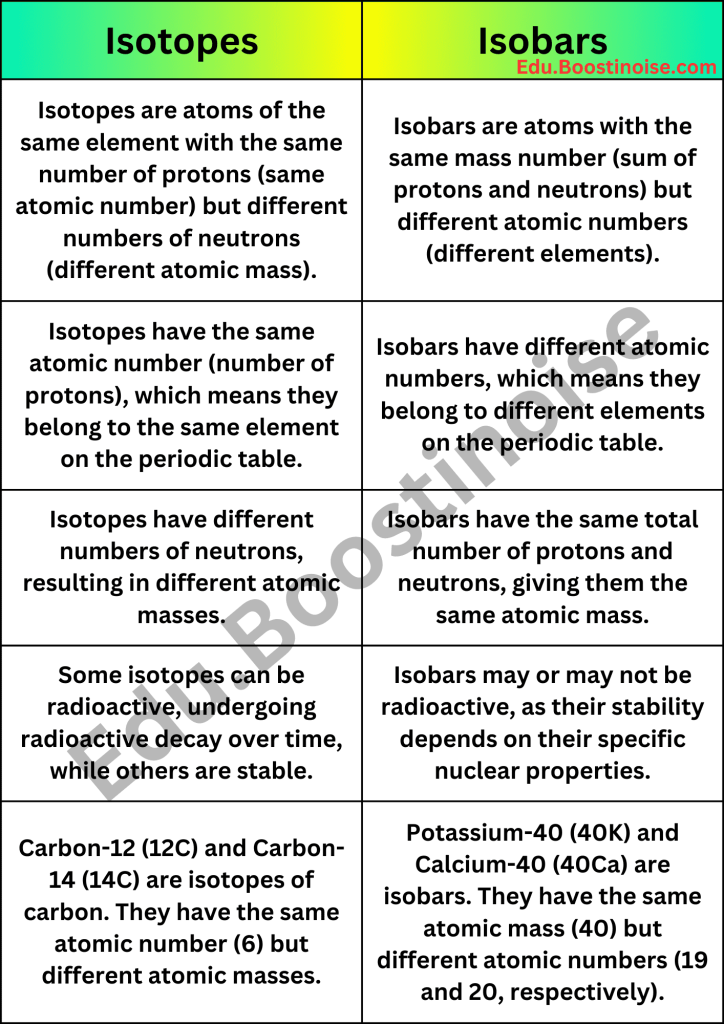
Difference between isotopes and isobars
The differences between isotopes and isobars given in below
Definition: The Isotopes are atoms of the same element with the same No. of protons but different numbers of neutrons, while isobars are atoms of different elements with the same mass number.
Chemical Properties: Isotopes have a chemical properties due to the same number of electrons and protons, whereas isobars have different chemical properties due to the different numbers of protons and electrons.
Natural vs. man-made: Isotopes can occur naturally or be man-made created, while isobars are typically artificially created in particle accelerators or nuclear reactors.
Atomic Mass vs. Atomic Number: Isotopes have different atomic masses, whereas isobars have different atomic numbers.
Defination of Isotopes
Iotas of similar component with similar number of protons however various quantities of neutrons are called isotopes. Protons have a positive charge, while neutrons are unbiased. The amount of protons chooses the part’s personality, while the amount of neutrons impacts its atomic mass.
Properties of Isotopes
Isotopes of a component share similar synthetic properties since they have similar number of electrons and protons. Nonetheless, because of various quantities of neutrons, isotopes might have somewhat unique actual properties like nuclear mass and strength.
Examples of isotopes
Definition of Isobars
Isobars are the atoms from different elements that have the same mass number. The mass no. is the total no. of protons and neutrons in an atom’s center. Isobars can have different atomic numbers, which means they belong to different elements, but their total number of protons and neutrons is equal.
Properties of Isobars
Isobars have different behaviors because they have different amounts of particles called protons and electrons. The number of protons decides an atom’s atomic number, which then decides how it behaves in chemicals. Even though isobars might have the same total particles, they are different chemically.
Examples of Isobars
Let’s take an example: Carbon-14 and Nitrogen-14 are isobars. Nitrogen-14 has 7 Protons and 7 Neutrons and C-14 has 6 protons and 8 neutrons. Even though they both weigh the same (mass number 14), they are actually different elements and behave differently in chemistry.
Application of Isotopes and Isobars in Various Fields
Isotopes and isobars find practical applications in a wide range of scientific disciplines and industries. Let’s explore some of these applications:
Medicine and Healthcare: Isotopes play a crucial role in medical imaging, diagnostics, and therapy. Radioactive isotopes, such as technetium-99m, are used in techniques like Single-Photon Emission Computed Tomography (SPECT) and Positron Emission Tomography (PET) scans to visualize organs and detect abnormalities. Isotopes are also utilized in radiation therapy to target and destroy cancer cells.
Archaeology and Anthropology: Isotopic analysis of archaeological artifacts, human stays, and ancient substances presents precious insights into past civilizations, migration patterns, and nutritional habits. Carbon dating of archaeological sites, artifacts, and historical sites is based primarily on the decay of C-14 isotopes.
Environmental Studies: Isotope analysis is a powerful tool in studying environmental processes. It helps trace the origin and movement of pollutants, track nutrient cycles in ecosystems, and understand climate change dynamics. Isotopes of elements like oxygen, carbon, and nitrogen are used to investigate processes such as groundwater flow, carbon cycling, and nutrient uptake in plants.
Geology and Earth Sciences: Isotopes have significant applications in geochronology, the study of geological time. Radiometric dating methods utilize the decay of isotopes to determine the ages of rocks, minerals, and geological formations. Isotopic evaluation also aids in knowledge the formation and evolution of Earth’s crust, volcanic activities, and the motion of tectonic plates.
Energy and Nuclear Power: Isobars, particularly those with unstable nuclei, have applications in nuclear power generation. Isotopes like uranium-235 and plutonium-239 undergo nuclear fission, releasing a remarkable amount of electricity. This process is harnessed in nuclear reactors to produce electricity on a large scale.
Industrial and Agricultural Applications: Isotopes are employed in various industrial processes and quality control measures. Stable isotopes are used as tracers to monitor chemical reactions, study catalysis, and analyze the composition of materials. In agriculture, isotopic analysis aids in understanding nutrient uptake by crops, optimizing fertilizer usage, and assessing water resource management.
conclusion
In conclusion, in chemistry, isotopes and isobars are distinct concepts. Isobars are atoms of different elements with the same mass number, whereas isotopes are atoms of the same element with different numbers of neutrons. Understanding these distinctions is fundamental for appreciating the way of behaving of components and their different structures. By concentrating on isotopes and isobars, researchers gain important experiences into atomic cycles, compound responses, and the construction of issue.
FAQ
Most frequent questions and answers
Isotopes have the same wide variety of protons but different numbers of neutrons inside atoms of the equal detail. Isobars, then again, have the same mass wide variety but belong to exclusive factors with unique atomic numbers.
Isotopes can occur naturally, while isobars are typically artificially created in controlled laboratory settings.
Isotopes have similar chemical properties due to the same number of protons and electrons. In contrast, isobars have unique chemical residences because they have exclusive numbers of protons and electrons.
Isobars such as uranium-235 and uranium-238 are needed to maintain nuclear chain reactions and to generate electricity in nuclear power plants.

