Main Difference – Homogeneous vs. Heterogeneous Mixtures
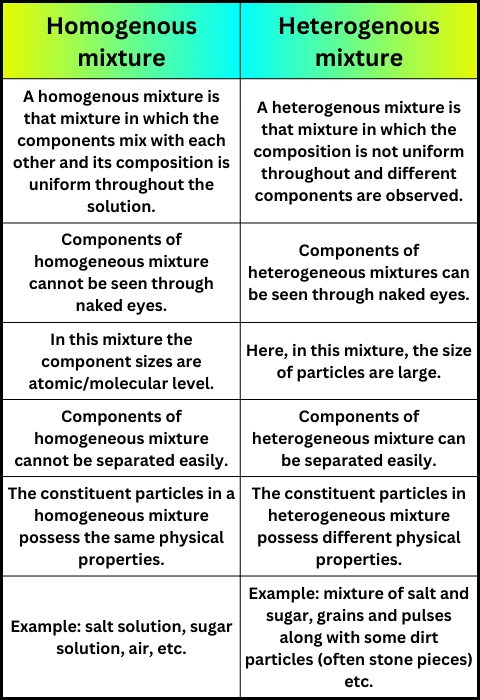
A mixture is a combination of different substances that retain their properties and can be separated by physical means. These different particles do not alter any chemical changes as part of the mixture. Mixtures are divided into two main categories: homogeneous mixtures and heterogeneous mixtures. The terms homo and hetero indicate a more obvious distinction between homologous and heterogeneous compounds.
The prefix homo indicates unity while odd indicates inequality. Homogeneous mixtures have the same properties throughout the system, as opposed to heterogeneous mixtures. The particles in heterogeneous are arranged randomly while the particles in homogeneous mixtures are arranged in a more ordered manner, giving a homogenous structure mixture is a combination of different substances that retain their properties and can be separated by physical means. These different particles do not alter any chemical changes as part of the mixture. Mixtures are divided into two main categories: homogeneous mixtures and heterogeneous mixtures. The terms homo and hetero indicate a more obvious distinction between homologous and heterogeneous compounds.
Homogeneous Mixtures :
Homogeneous mixtures are uniform mixtures where the components are evenly distributed at a molecular level, resulting in a consistent composition throughout is called Homogeneous Mixtures
Heterogeneous Mixtures :
Heterogeneous mixtures are non-uniform mixtures where the components are not evenly distributed, and distinct regions with varying compositions are visible is called Heterogeneous Mixtures.