The calorific worth of a strong or non-unpredictable fluid fuel is not entirely set in stone with a bomb calorimeter.
Principle: A known load of the example (solid or fluid fuel) is sung totally in the overabundance of oxygen. Encompassing water a calorimeter assimilates the freed heat. Thus the heat liberated during fuel combustion is equal to the heat absorbed by water and copper calorimeter. The higher calorific value of the fuel is calculated from the data.
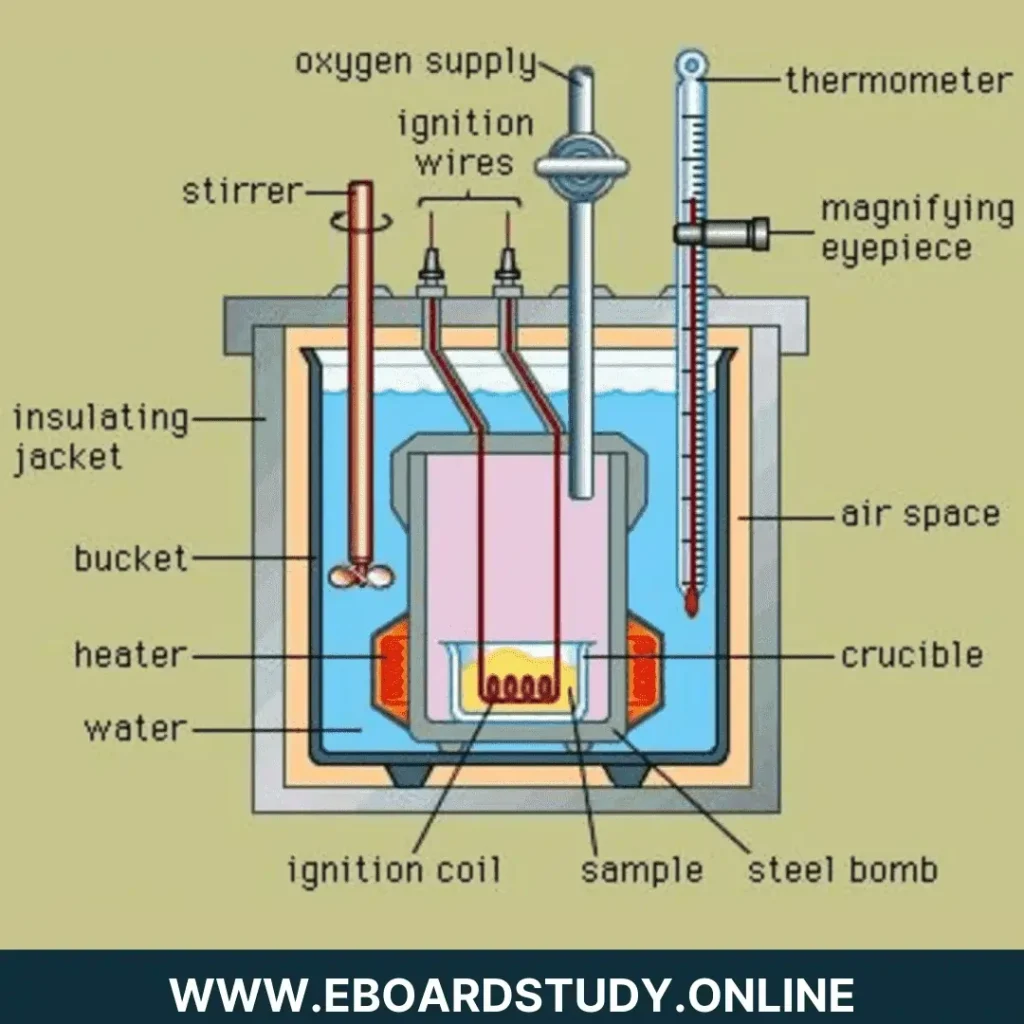
Construction: A basic bomb calorimeter comprises of major areas of strength for a treated steel vessel, which is erosion-safe and fit for enduring high strain. It is called a bomb. It is furnished with a cover, which can be in a bad way firmly to the group of bombs to make an ideal gas-tight seal. The cover is given two hardened steel cathodes and an oxygen bay valve. A little ring is joined to one of the terminals which goes about as a help for the cauldron. The bomb is put in a copper calorimeter, which is encircled via air-coat and water-coat to forestall the deficiency of intensity because of radiation. The calorimeter is furnished with an electrically worked stirrer and a Beckmann’s thermometer, which can peruse precisely temperature distinction up to 1/100th of a degree centigrade
Calculations:
Let, the weight of the fuel test taken = m gm
Weight of water measured in calorimeter = W gm
Water likeness calorimeter, bomb, thermometer, stirrer, and so forth. = w gm
Introductory temperature of water = t1 °C
Last temperature of water = t2 °C
G.C.V. of fuel = θ cal/gm
Then, heat freed by burning of fuel = m.θ
Furthermore, heat is consumed by water, calorimeter, and so forth. = (W + w)( t2 – t1)
Presently, heat retained = heat freed
m.θ = (W + w)( t2 – t1)
θ = (W + w)( t2 – t1)/m
For additional exact outcomes, the following remedies ought to be integrated:
A) Corrosive revision, tA
B) Circuit wire adjustment, tF
C) Cotton string adjustment, tT
D) Cooling adjustment, tC
In like manner, the above condition should be adjusted as follows:
G.C.V., θ = [(W + w)( t2 – t1+ tC) – (tA + tF + tT)]/m
N.C.V. = G.C.V. – 9 x H/100 x 587
N.C.V. = G.C.V. – 0.09 x H x 587
Where, H = % of hydrogen in the fuel.
The above rectifications in the equation can be made sense of as follows:
Corrosive amendment (tA): During the start sulfur and nitrogen (if present) in the fuel are oxidized to sulphuric corrosive and nitric corrosive separately alongside the advancement of intensity.
S + O2 → SO2
2 SO2 + O2 + 2 H2O → 2 H2SO4 ; ΔH = – 144 KCal
2 N2 + 5 O2 + 2 H2O → 4 HNO3 ; ΔH = – 57.16 KCal
the modification should be made as follows
3.6 cal ought to be deducted for every ml of N/10 H2SO4.
1.43 cal ought to be deducted for every ml of N/10 HNO3.
Intertwine wire remedy (tF): The deliberate intensity additionally incorporates the intensity given by the start of the wire and consequently it should be deducted from the absolute intensity freed.
Cotton string amendment (tT): The rectification of the cotton string utilized for terminating the fuel is determined from the heaviness of the dry cotton string utilized and it ought to be deducted from the complete intensity freed.
Cooling adjustment (tC): Assuming the time taken for the water in the calorimeter to cool from the most extreme temperature to room temperature is x minutes and the pace of coolingdt°/min, then, at that point, the cooling rectification will be ‘x.dt’. This ought to be added to the climb in the temperature ( t2 – t1).

